Physicists led by Roland Vester of the University of Innsbruck have taken a closer look at the competition between two important reaction mechanisms in organic chemistry in the laboratory. The detailed investigation of the reaction dynamics of a reaction complex consisting of nine atoms has been unique so far. In doing so, scientists are advancing on a scale that enables applications in many areas of chemistry.
With laboratory experiments, two-time ERC Prize winner Roland Vester from the Institute for Ion Physics and Applied Physics at the University of Innsbruck tries to take a fresh look at chemical reactions and better understand their dynamics. The reactions of small molecules are well understood today. As soon as more than four atoms are involved, it becomes difficult for both theory and experiment to describe the course of the reaction in detail. Roland Vester and his team have created a unique experiment with which molecules can react with ions and observe them. For the first time, scientists have succeeded in accurately describing the atomic dynamics of a so-called nucleophilic substitution reaction. A few years ago, the research group investigated the competition between two chemical reactions in organic compounds. In a vacuum chamber, the researchers brought these molecules to collide with charged particles from the chemical group of halogens, such as fluorine, iodine and chlorine. For the first time, direct observation has shown that with larger molecules the elimination reaction gains the upper hand and the substitution reaction disappears at some point.
high dimensional reaction dynamics
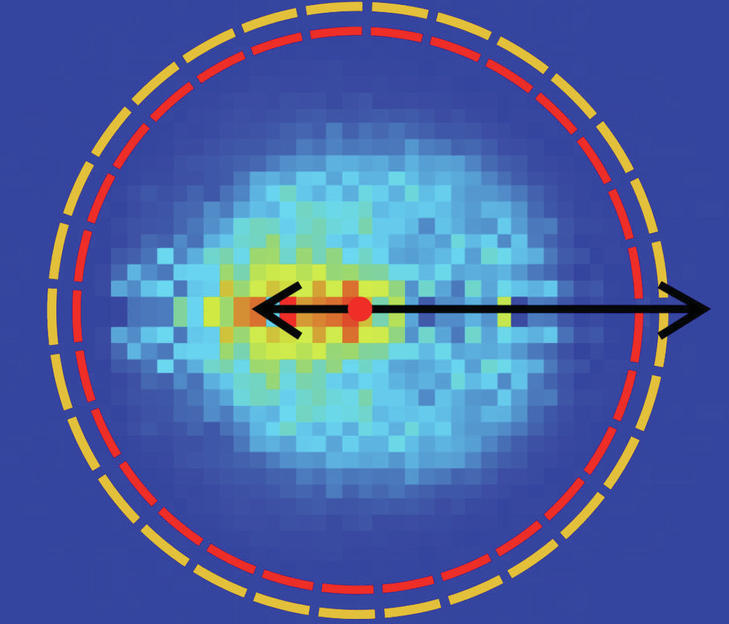
It was not yet known what would happen in the size range where both reactions are equally important. To investigate this, further theoretical work was necessary, which was carried out in recent years by researchers led by Gabor Czako at the University of Sejed in Hungary. They have calculated a so-called Born–Oppenheimer potential surface, along which the course of a chemical reaction of a molecule consisting of eight atoms with a halogen ion can be described in detail. It is noteworthy that the tested reaction occurs in 21 dimensions. The theoretically determined possible scenario provides insight into how individual atoms may move in this high-dimensional space during a chemical reaction. From this, Innsbruck scientists working with Eduardo Carrascosa, Jennifer Meyer and Roland Vester were able to make a very accurate prediction of the spatial direction in which the reaction products would fly in their experiment. Because they measure the angle and speed at which ions hit a detector. “And our data shows that we measured exactly the same way we calculated theory without knowing the experimental data,” says Wester happily. “With so many atoms and so high-dimensionality, this hasn’t been achieved yet.”
Scientists have thus widely described the chemical reaction, which consists of two different reaction mechanisms, both theoretically and experimentally. With this work, which is now published in the journal Nature Chemistry, the Innsbruck physicists come to a field for detailed investigation of reaction dynamics with respect to the number of atoms involved, enabling applications in many areas of complex chemical reactions. makes.

Internet fan. Alcohol expert. Beer ninja. Organizer. Certified tv specialist. Explorer. Social media nerd.