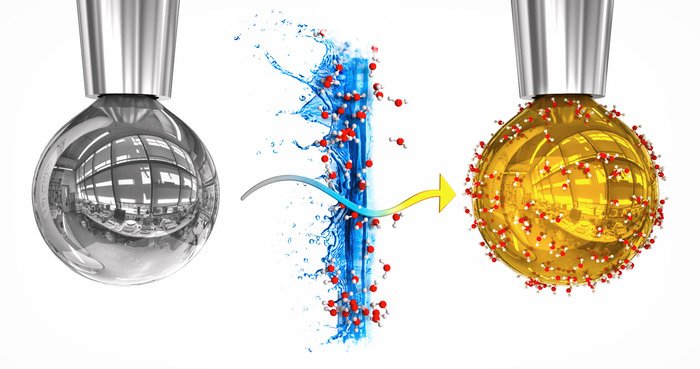
Miraculous Transformation: For the first time, researchers have described the properties of water metal without high pressure and at room temperature. This was achieved through a nifty trick: They condense water vapor onto a drop of liquid alkali metals in a vacuum. The migration of electrons from the metal to the thin layer of water gave the metal conductivity and appearance for a short period of time: the drop took on the luster of gold.
Metals have good conductivity because localized outer electron The atom can move freely in the lattice. It is different with distilled water: because the outer electrons of the atoms involved are tightly bound, pure water is a nearly perfect insulator. To make it conductive and thus metallic, the water molecules would have to be strongly compressed. The high pressure then brings the orbitals of the outer electrons so close to each other that they overlap. Due to the increased electron density, some of the electrons move away and thus allow the flow of current.
The problem, however: “In the case of pure water, the pressure required for this is estimated at 48 megabars – this is beyond our experimental possibilities today and probably only occurs in the cores of large planets or stars,” explains Philip Mason. Czech Academy of Sciences in Prague and colleagues. In fact, there is some evidence that hydrogen, helium, and possibly even water inside Jupiter Could be metal.
Alkali metals and water – a delicate combination
But there is another way, as the researchers are now proving in an experiment. The starting point for this was the idea that the electron density of water could also be increased by doping – by injecting additional electrons from another material. It was also known from earlier experiments that alkali metals would in principle be suitable as electron donors.
When it comes to the combination of alkali metals and water, there’s an important hurdle—as most of you know from school: “If you throw a lump of sodium in water, you don’t get metallic water, But an immediate and violent explosion,” explains Mason’s colleague Pavel Jungwirth. “To avoid this violent and rather counterproductive reaction, we did the whole thing the other way around: We put water in the metal.” The amount of water was so low that there was no explosion.
the drop turns shiny gold
Specifically, the experiment works like this: A tiny drop of a sodium-potassium solution is ejected from a fine nozzle into a high-vacuum sample chamber. The silver drop rises for about ten seconds before detaching from the nozzle and falling down. While the drop is forming, the researchers inject water vapor into the chamber. Some of the water molecules are deposited on the surface of the droplets and form a layer of water that is thicker than about 80 molecule layers per second.
The interesting thing about it is that as soon as the first water molecules are deposited on the metal droplet, its color changes. “The sodium-potassium drop has a golden glow, which is very impressive,” reports co-author Robert Seidel of the Helmholtz Center for Materials and Energy in Berlin. The metallic gold glow persists for about five seconds, before the drop’s color eventually changes from bronze and purple to white.
free electrons in the water layer
This color change indicates a decisive change in the electrochemical behavior of the water layer. As confirmed by spectroscopic analyses, non-conductive water becomes conductive when glowing – it assumes metallic properties. “Our observations confirm that the golden hue and its characteristic luster reflect the metallic nature of the aqueous surface layer,” Mason and his colleagues wrote.
This can be measured by charge fluctuations, the presence of so-called plasmons, and the fact that there is a conduction band with a width of about 1.1 electron volts, as the researchers describe. Both indicate that there are now freely moving electrons in the water. They come from the alkali metal and are transferred to the aqueous layer after the water molecules are deposited. By measurement, the density of these extra electrons during the gold phase is about five trillion electrons per cubic centimeter.
This is how water conversion works.© Thunderf00t
metal without high pressure
This experiment thus proves what was previously believed to be impossible: water can be made metallic even without extreme pressure. In the drop experiment, this condition, which is unusual for pure water, lasted a few seconds, as the researchers report. Then, however, the chemical reaction of alkali metals with water forms white alkali hydroxide.
“Our study not only shows that metallic water may indeed have originated on Earth, but also characterizes the spectroscopic properties associated with its beautiful golden metallic luster,” Seidel says. This will make it possible to conduct research on the properties and behavior of metallic water in the future even without high pressure. (Nature, 2021; doi:10.1038/s41586-021-03646-5)
Source: Helmholtz Center Berlin for Materials and Energy, Institute of Organic Chemistry and Biochemistry of the Czech Academy of Sciences

Internet fan. Alcohol expert. Beer ninja. Organizer. Certified tv specialist. Explorer. Social media nerd.